Covid-19 vaccine trials start in Blackpool next week
and live on Freeview channel 276
The phase three vaccine trials will start on Monday September 28, and 500 volunteers will be able to visit "either Layton Medical Centre or Blackpool Victoria Hospital" to take part.
Clinics will be held on Mondays, Wednesdays, and weekends at the surgery, and Monday to Friday at the Vic, in the hope as many people as possible are able to participate.
Advertisement
Hide AdAdvertisement
Hide AdDr Rebecca Clark, GP and partner at Layton Medical Centre, will be the principal investigator of the trial, playing a key role in developing research in the fight against Covid-19.
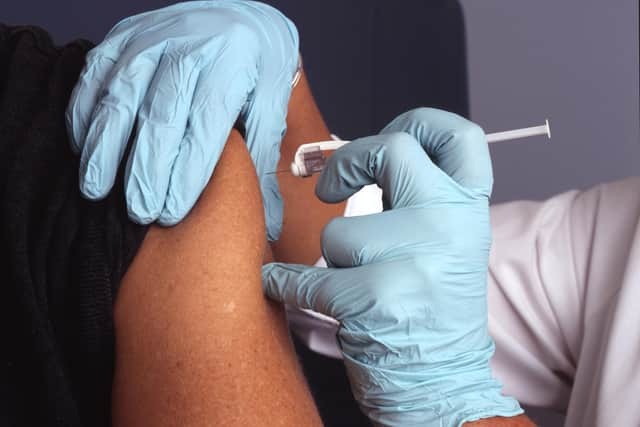

She said the study was "incredibly exciting" and urged Fylde coast residents to volunteer.
"This is really important for Blackpool, it's taken a lot of hard work to secure this for the Fylde coast," Dr Clark said.
"The only way out of this pandemic is with a vaccine. The purpose of it is to protect as many people as possible and allow them to build an immunity to the virus.
Advertisement
Hide AdAdvertisement
Hide Ad"It's important to remember we aren't out of the woods yet."


In the double-blind trial, 50 per cent of participants will receive a Covid-19 vaccine, and the other 50 per cent will receive a saline placebo.
Dr Clark reassured anyone considering participating that "you could not get Covid-19 from the vaccination."
She continued: "If 500 people go into the study, there could potentially be 250 residents of the Fylde coast who may well be protecting the NHS over the winter months.
Advertisement
Hide AdAdvertisement
Hide Ad"We could have a cohort of people who hopefully have some degree of protection."


From October 5, the trials will also take place at Blackpool Victoria Hospital's Patient Recruitment Centre (PRC), which continues to carry out Covid-19 research with patients.
One of only five centres in the country, it was set up to enable patients to take part in clinical studies and help to decrease the time it takes to roll out late-phase trials, such as the Blackpool Covid-19 vaccine trial.
The Covid-19 vaccine study will be the first to run through the PRC at the Vic since it launched in May.
Advertisement
Hide AdAdvertisement
Hide AdDr Angela Parker, research and development manager at the centre, said the vaccine trial was in "phase three, so larger numbers of people are needed."
"I feel elated that we've secured this for our residents, it's a fantastic opportunity for people," Dr Parker said.
"We have been working extremely hard in the Trust, and we have offered a lot of recovery trials to in-house patients.
"These vaccine trials aren't scary, and everything is fully explained so people can make their own informed decisions.
Advertisement
Hide AdAdvertisement
Hide Ad"But a vaccine is our route out of this, and we could potentially have one here that may help.
"The sooner we are out of this, the sooner we can go back to life as normal."
The NHS was unable to give the Gazette any further information about the vaccines until the first day of the trial.
Anyone aged between 18 and 64 from all ethnic backgrounds are able to participate, as long as you have not tested positive for Covid-19 or virus antibodies in the past.
Advertisement
Hide AdAdvertisement
Hide AdPregnant women or women trying to conceive are also unable to participate.
Residents from all areas of the Fylde coast are able to take part, including those under the local lockdown restrictions in Wyre and Fylde, Dr Clark said.
If you want to be involved in the vaccine trial, email Dr Clark at [email protected] to express your interest.
What is a phase three vaccine trial?
Clinical development is a three-phase process. During phase one, small groups of people receive the trial vaccine.
Advertisement
Hide AdAdvertisement
Hide AdIn phase two, the study is expanded and the vaccine is given to people who have characteristics (such as age and physical health) similar to those for whom the new vaccine is intended.
In phase three, the vaccine is given to thousands of people and tested for efficacy and safety.
What are the stages for development of new vaccines?
The general stages of producing a new vaccine from start to finish are:
- Exploratory stage
- Pre-clinical stage
- Clinical development
- Regulatory review and approval
- Manufacturing
- Quality control