Blackpool Covid-19 vaccine trials: what to expect if you take part
and live on Freeview channel 276
Phase three trials of the Novavax vaccine will begin next week at Layton Medical Centre and Blackpool Victoria Hospital's Patient Recruitment Centre (PRC), led by principal investigator Dr Rebecca Clark and researcher Dr Angela Parker respectively.
Earlier this week, it was revealed the Blackpool trials would be open to 18 to 64 year olds, but confidence in its safety and "successful results" in phase one and two has resulted in the age being extended to 84 years old.
Advertisement
Hide AdAdvertisement
Hide AdDr Rebecca Clark, principal investigator of the trial, GP and partner at Layton Medical Centre, said a vaccine was our "route out" of the coronavirus pandemic.
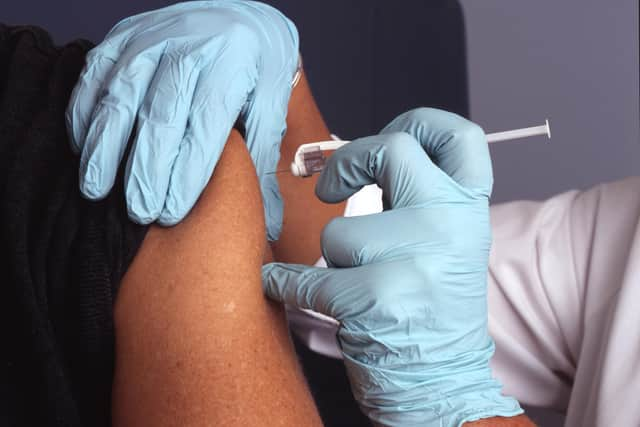

She explained: "This is really important for Blackpool, it's taken a lot of hard work to secure this for the Fylde coast.
"The only way out of this pandemic is with a vaccine. The purpose of it is to protect as many people as possible and allow them to build an immunity to the virus.
"We can now extend the trials to elderly people, because the phase one and two trials showed the vaccine was safe enough to do so. We're now inviting anyone up to the age of 84 to take part."
Advertisement
Hide AdAdvertisement
Hide Ad"Around 600" people have already volunteered to take part in the vaccine trials.
It was hoped that by taking part, Fylde coast participants could help to protect the NHS during the winter months, and protect other residents susceptible to catching viruses.
Professor Paul Heath, Novavax phase 3 trial chief investigator and professor of paediatric infectious diseases at St George’s University Hospital said: “This is only the second phase 3 vaccine study to be initiated in the UK, and the first phase 3 study with the Novavax vaccine anywhere in the world, which shows the importance that has been placed on rapidly finding a solution for this urgent public health need.
"The vaccine has successfully gone through its early safety trials and we’re extremely encouraged by its performance so far."
What is the vaccine?
Advertisement
Hide AdAdvertisement
Hide AdThe Novavax vaccine is made up of a combination of nanoparticles, containing an engineered Covid-19 spike protein and the plant chemical-based adjuvant Matrix-M. Adjuvants are ingredients added to vaccines to boost immune responses and stimulate high levels of neutralising antibodies.
Half the participants will receive the trial Covid-19 vaccine, delivered in two doses, and half will receive a saline placebo, also delivered in two doses. Nobody taking part will know if they are receiving the vaccine or a placebo.
What is the purpose of the vaccine?
The vaccine has been developed in a bid to help people build an immune response to Covid-19.
How many doses will I need?
Volunteers can expect to make "around six visits to their local trial (either Layton Medical Centre or Blackpool Vic) over 13 months."
Can I get Covid-19 from the vaccine?
Advertisement
Hide AdAdvertisement
Hide AdNo, Dr Rebecca Clark confirmed there is "absolutely no risk" of getting the virus by taking part in the vaccine trials.
Will I get paid if I take part?
No, but travel expenses are paid for.
Who can take part?
Anybody between the ages of 18 and 84. People from Black, Asian and Ethnic minority backgrounds are encouraged to take part, "to better understand the effectiveness of vaccine candidates and help find a vaccine that works for as many people as soon as possible."
Who is unable to take part?
Pregnant women or women trying to conceive, anyone under 18, and anyone who has tested positive for Covid-19 or the virus' antibodies in the past.
Can I still get my flu vaccine if I take part?
Yes, Dr Rebecca Clark confirmed that participants can still have a flu injection.
How do I get involved?
Advertisement
Hide AdAdvertisement
Hide AdTo take part in the trials in Blackpool, email [email protected]What is a phase three vaccine trial?
Clinical development is a three-phase process. During phase one, small groups of people receive the trial vaccine.
In phase two, the study is expanded and the vaccine is given to people who have characteristics (such as age and physical health) similar to those for whom the new vaccine is intended.
In phase three, the vaccine is given to thousands of people and tested for efficacy and safety.
What are the stages for development of new vaccines?
The general stages of producing a new vaccine from start to finish are:
- Exploratory stage
- Pre-clinical stage
- Clinical development
- Regulatory review and approval
- Manufacturing
- Quality control